On the Monte Carlo simulation of neutron-induced direct and indirect DNA damage to estimate neutron relative biological effectiveness
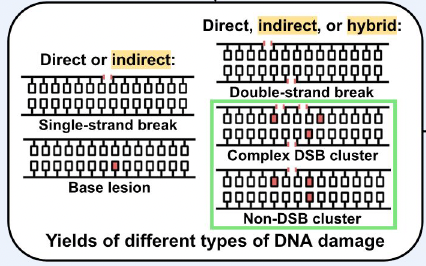 Image credit: James Manalad
Image credit: James ManaladAbstract
Introduction The exposure of patients undergoing high-energy radiotherapy (RT) to secondary neutron radiation poses a risk for iatrogenic second cancer induction. However, this risk is poorly understood. With a goal of improving our understanding of the risk, our research program is studying the biophysical mechanisms underlying neutron-induced carcinogenesis by investigating, among other topics, the roles of direct and indirect action in the formation of DNA lesions. We recently explored the role of neutron direct action [1], but a study on neutron indirect action was outstanding. Therefore, the aims of this project were to (i) estimate the energy-dependent relative biological effectiveness (RBE) of neutrons for inducing double-strand-break (DSB)-containing clusters (complex DSB clusters) due to the combined effects of direct and indirect action (referred in this work as combined action), and (ii) to determine whether such an RBE estimation may be used as a measure of neutron carcinogenic risk by comparing our results with the established energy-dependent neutron radiation weighting (wR) [2] and quality (Q) [3] factors. Materials & Methods The existing simulation pipeline of our research group for the direct action of ionizing radiation (built using the TOPAS and TOPAS-nBio frameworks and incorporating a custom geometric DNA model) [1] was extended to incorporate an implementation of indirect action with benchmarking against published in vitro and in silico experiments. Using our updated pipeline, we simulated irradiations of monoenergetic neutrons and reference 250 keV X-rays at various depths in a human body phantom (ICRU sphere [4]). The DNA damage yields (i.e., number of lesions) induced in our entire DNA model of 6.3 Gbp [1] by our simulated irradiations were used to quantify the energy-dependent neutron RBE for causing complex DSB clusters across the energy range relevant to RT (1 eV to 10 MeV). Discussion & Conclusions Our results show that most neutron-induced DNA damage events are isolated simple lesions due to indirect action (single-strand breaks and base lesions, see Figure 1), while most complex DSB clusters are hybrid in nature (see Figure 2). Our calculated neutron RBE values related to the induction of complex DSB clusters are found to be smaller in magnitude than previously-reported values [1, 3] that considered only direct action (see Figure 3), despite our larger yields (see black lines in Figure 1). We are currently analyzing data if this is due to the much higher density of lesions in neutron-induced damage clusters, resulting in more lesions contributing to each cluster and thus, less clusters overall compared to the reference X-rays. In conclusion, our findings demonstrate that (i) indirect action plays an important role in neutron-induced DNA damage and (ii) the energy-dependent RBE curve for neutron combined action estimated from yields of complex DSB clusters alone reaches a peak at a similar energy to the established radioprotection factors for neutron radiation, but with a lower magnitude.